NOTICIAS
How incidents with medicines are managed in the EU – a ten-year analysis by EMA
published by EMA News 23/09/2020. The EU medicines network is supported by a robust regulatory framework with defined processes and clear responsibilities in place to handle public health incidents, according to a 10-year analysis of the European Union incident...
Regulating medical devices from 1 January 2021 for the UK medical devices market
Published 1 September 2020 From: Medicines and Healthcare products Regulatory Agency New rules for January 2021 The UK has left the EU, and the transition period after Brexit comes to an end this year. From 1 January 2021 the Medicines and Healthcare products...
Últimas vagas para a 6ª Edição da formação sobre Gestão de Reclamações, Devoluções, Falsificados e Recolhas de Mercado
Últimas vagas disponíveis para a 6ª Edição Gestão de Reclamações, Devoluções, Falsificados e Recolhas de Mercado, do próximo dias 27 de outubro , conduzido pela conceituada especialista Dra. Sónia Rei , da Hikma . Nesta iniciativa oferecemos uma formação para...
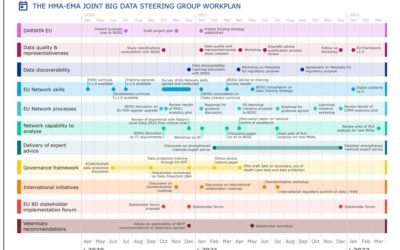
Making best use of big data for public health: publication of the Big Data Steering Group workplan for 2020-21
PUBLISHED BY EMA. EMA EUROPE News 14/09/2020 The Big Data Steering Group set up by EMA and the Heads of Medicines Agencies (HMA) has published its workplan which sets actions to be delivered in 2020-21. With the European Medicines Regulatory Network focused on...
Guidelines 07/2020 on the concepts of controller and processor in the GDPR
Guidelines 07/2020 on the concepts of controller and processor in the GDPR Version 1.0 Adopted on 02 September 2020 This document seeks to provide guidance on the concepts of controller and processor based on the GDPR’s rules on definitions in Article 4...
NOVAS DATAS para a formação sobre as BOAS PRÁTICAS DE DISTRIBUIÇÃO DE MEDICAMENTOS E DISPOSITIVOS MÉDICOS
Dada a elevada procura , abrimos novas datas para as formações sobre: Gestão de Reclamações, Devoluções, Falsificados e Recolhas de Mercado Lisboa, 27 de outubro de 2020 Boas Práticas de Distribuição de Dispositivos Médicos Lisboa , 11 e 12 de novembro de 2020...
FDA NEW GUIDANCE : “Biological evaluation of medical devices
The scope of this document and accompanying attachments is limited to the biological evaluation of sterile and non-sterile medical devices that come into direct or indirect contact with the human body. This document specifically covers the use of ISO 10993-1 but also...
NEW MDSAP DOCUMENT: mdsap audit approach.
The intention of the Medical Device Single Audit Program (MDSAP) is to allow competent auditors from MDSAP recognized Auditing Organizations (AOs) to conduct a single audit of a medical device organization’s quality management system that will satisfy the requirements...
The European Commission has published a frequently asked questions document (FAQs) on UDI System
The European Commission has published a frequently asked questions document (FAQs) on UDI System The existing regulatory framework on medical devices dates back to the 1990s and consists of three Directives. Two new Regulations (Regulation (EU) 745/2017 on...
COSMETIC BORDELINE MANUAL. SEPT 2020
MANUAL OF THE WORKING GROUP ON COSMETIC PRODUCTS (SUB-GROUP ON BORDERLINE PRODUCTS) ON THE SCOPE OF APPLICATION OF THE COSMETICS REGULATION (EC) NO 1223/2009 (ART. 2(1)(A)) VERSION 5.2 (SEPTEMBER 2020) The clear determination of the scope of application of...
EC: Updated version 18 of Q&A on safety features for medicinal products for human use
On August 12, 2020, the European Commission published version 18 of the Q&A on safety characteristics for medicinal products. The document, which has now grown to 34 pages, was thus the second update this year New are the questions 4.6, 5.12, 5.13 and 6.9, which...
Good reliance practices in regulatory decision-making for medical products: high-level principles and considerations
The World Health Organization (WHO) supports the implementation of reliance on other regulators’ work as a general principle in order to make the best use of available resources and expertise. This principle enables leveraging the output of others whenever possible...
CONTRATAÇÃO PÚBLICA – GUIA PRÁTICO PARA PROFISSIONAIS
O presente guia destina-se essencialmente aos profissionais que, lidando com contratos públicos no seio de autoridades adjudicantes na União Europeia, são responsáveis por planear e levar a cabo uma aquisição conforme, eficiente e com uma boa relação qualidade/preço...
EMA new guideline on the Quality of Water for Pharmaceutical Use
The European Medicines Agency issued a new guideline on the quality of water for pharmaceutical use that will replace its nearly two-decades-old guidance and position statement on water quality when it takes effect in February 2021 This document is...
Launch of public consultation on joint network strategy to 2025
EMA and the Heads of Medicines Agencies (HMA) have developed a joint strategy for the next five years that is released for a two-month public consultation today. The draft strategy details how the European medicines agencies’ network can continue to enable the supply...
FDA New Guidance on Multiple Function Device Products
Medical products may contain several functions, some of which are subject to FDA’s regulatory oversight as medical devices, while others are not. Section 3060(a) of the 21st Century CuresAct (Cures Act) amended the Federal Food Drug, and Cosmetic Act (FD&C Act) to...
Novos pedidos de AIM: Uma formação muito útil e com excelentes exercícios práticos
Decorreu nos dias 22 e 23 de julho de 2020, a 1ª edição da formação sobre os Novos pedidos de AIM , conduzida pelo especialista Dr Selmo Pinto,MRP/DCP Process Manager,do INFARMED Uma combinação de teoria e exercícios práticos, para adquirir os conhecimentos ...
Formação sobre Promo review Compliance & Best practices 2020: Mais uma edição marcada pelo sucesso
Teve lugar no dia 21 de Julho 3ª edição da formação exclusiva da Formiventos sobre PROMOTIONAL REVIEW COMPLIANCE & BEST PRACTICES conduzida pelo especialista DR Ricardo Andrade, Managing Director , da OWL PHARMA CONSULTING, bajo o lema “Como conseguir a...
Cannabis and Cannabis-Derived Compounds: Quality Considerations for Clinical Research. FDA Guidance for Industry
This guidance outlines FDA’s current thinking on several topics relevant to clinical research related to the development of drugs containing cannabis or cannabis-derived compounds. Cannabis and cannabis-derived compounds that may be used in drug manufacturing...
Veterinary Medicines Regulation HIGHLIGHTS
The European Union create the newsletter for the Implementation of the Veterinary Medicines Regulation (VMP-Reg) Programme. The Regulation will become applicable in January 2022 after a 3-year implementation period. In 2019, EMA focussed on preparing a number of...
Clinical evaluation assessment report template .July 2020
This template applies to MDR Annexes IX section 4 and Annex X section 3. It also applies to assessments of technical documentations on a sampling basis for class IIa/IIb devices in accordance with Annex IX sections 2.3 and 3.5 and Section 10 of Annex XI(A). Aspects...
BOAS PRÁTICAS de DISTRIBUIÇÃO de MEDICAMENTOS: “Muito útil, dado por alguém com muito conhecimento.Temas muito bem estruturados”
Reconheciendo a relevância do cumprimento das Boas Práticas de Distribuição na indústria farmacêutica, a Formiventos organizou a edição número 12 do Curso : BOAS PRÁTICAS de DISTRIBUIÇÃO de medicamentos de uso humano e de substâncias ativas Nesta nova edição foram...
Vagas esgotadas para a formação sobre PROMOTIONAL REVIEW
No próximo 21 de Julho , terá lugar a 3ª edição da formação exclusiva da Formiventos sobre PROMOTIONAL REVIEW COMPLIANCE & BEST PRACTICES conduzida pelo especialista DR Ricardo Andrade, Managing Director , da OWL PHARMA CONSULTING, bajo o lema “Como conseguir a...
The Value and Pricing of Innovative Medicines. @eupatientsforum
The European Patients Forum has released a new position paper entitled ‘The Value and Pricing of Innovative Medicines' . EPF’s position is based on the premise that health is a fundamental right and a critical investment in the well-being, economic development and...
ÚLTIMAS VAGAS na formação Boas Práticas de Distribuição de Medicamentos
A 13ª edição da formação sobre as BOAS PRÁTICAS de DISTRIBUIÇÃO de medicamentos de uso humano e de substâncias ativas, terá lugar os 14 e 15 de julho Para garantir condições ótimas de segurança, eficácia e conforto, o número de participantes é limitado HOT TOPICS...
Estratégia da Rede de Agências Europeias de Medicamentos até 2025 em consulta pública
publicado no site INFARMED 07 jul 2020 Os Chefes das Agências de Medicamentos (HMA na sigla inglesa) e a Agência Europeia de Medicamentos (EMA na sigla inglesa) lançaram uma consulta pública sobre a Estratégia da Rede de Agências Europeias de Medicamentos até...
Signal management: EMA e-learning course
These training materials seek to further improve understanding of signal management within the European medicines regulatory network and develop best practice in signal management. The course gives an overview of best practice in signal management. Main topics...
International regulators provide guiding principles for COVID-19 clinical trials
EMA Press release 01/07/2020. published by EMA ema.europa.eu EMA has endorsed a joint statement on prioritisation of COVID-19 clinical trials published by the International Coalition of Medicines Regulatory Authorities (ICMRA). Medicines regulators from around the...
Review and Update of Device Establishment Inspection Processes and Standards
FDA believes that uniformity in investigators’ approaches to inspections, both before and during, may inform firms’ preparation for the inspection and set baseline communication and timing expectations for each party. The processes and standards identified below...
APORMED publica Orientações para a retoma da interação entre os profissionais da indústria e os profissionais de saúde
Dada a elevada importância dos dispositivos médicos para os cuidados de saúde dos cidadãos portugueses e dos utentes do Sistema Nacional de Saude e por forma a garantir que os dispositivos médico são devidamente utilizados, é imprescindível a interação dos técnicos...
Medicines for Europe “LESSONS LEARNED FROM COVID-19”
After several months of crisis, we can discern some of the key lessons learned from COVID-19 for the future of pharmaceutical policy in Europe. Lessons learned from Covid-19 – Policy Paper
NOVAS REGRAS DE SEGURANÇA
Garantimos formações em condições ótimas de segurança, eficácia e conforto, com número de participantes limitado O número de presentes será muito reduzido – Máximo de 10 pessoas na sala A sala tem mais do dobro do espaço habitual, e cada participante terá um...
Estratégia Farmacêutica Europeia em consulta pública
Publicado no site Infarmed 8 jun 2020 A Comissão Europeia lançou a 16 de junho de 2020 uma consulta pública on-line sobre a estratégia europeia para o setor farmacêutico. Esta estratégia procura garantir o fornecimento de medicamentos seguros e acessíveis na Europa...
New Guidance on Good Clinical Practice (GCP)
In the context of this guidance, a remote / distant GCP inspection is defined as “the process of conducting inspections at a distance / virtually, supported by technology for communicating, sharing, reviewing, and developing documents and accessing systems, without...
Novas formações 2020
Apresentamos-lhe as novas formações para 2020 que esperamos sejam do seu interesse Formações em condições ótimas de segurança, eficácia e conforto, com número de participantes limitado
In-company BOAS PRÁTICAS DE DISTRIBUIÇÃO: Soluções à medida da sua empresa
De acordo com as GDP, o Diretor Técnico tem a responsabilidade de garantir os programas de formação inicial e contínua do pessoal no que respeita ao cumprimento das BOAS PRÁTICAS A regulamentação especifica a necessidade da formação do pessoal relativamente a: .-...
12ª Edição a formação BOAS PRÁTICAS de DISTRIBUIÇÃO, 14 e 15 de julho
Nos próximos dias 14 e 15 de julho de 2020 terá lugar a 12ª Edição da formação BOAS PRÁTICAS de DISTRIBUIÇÃO de medicamentos de uso humano e de substâncias ativas, conduzida pela reconhecida especialista Dra Sónia Rei, Head of Quality Systems & QP,...
Prospective dialogue between developers and regulators makes for better evidence generation
News 27/05/2020 EMA, in collaboration with other parties, has recently published two scientific articles outlining the importance of early interactions as an opportunity to improve the generation of evidence required for bringing innovation to patients. An article,...
O sector não pára e nós também não!
Estamos de volta ao trabalho após o encerramento causado pela pandemia COVID-19 Estamos a trabalhar para lhe voltar a oferecer as nossas iniciativas de formação, em condições otimas de segurança, eficácia e conforto. Nos próximos dias apresentaremos as datas do...
What is the state of play of the implementation of EUDAMED?
Currently, the EC database on medical devices, Eudamed2, is a secure web-based portal. It is a central repository for information on market surveillance exchanged between national competent authorities and the Commission. Its use is restricted to national competent...
EMA calls for high-quality observational research in context of COVID-19
26 May 2020 EMA/269353/2020 Media and Public Relations For observational studies of real world data in COVID-19, EMA calls for transparency for protocols and results, and collaboration between researchers, to ensure high-quality, powerful studies. High-quality...
Questions and Answers document regarding the Implementation of the new Manufacturer Incident Report (MIR) Form
Main changes introduced by the new MIR form The European Competent Authorities and Industry representative organisations agreed to use codified information on incidents (adverse events in IMDRF terminology) for the reporting of incidents in advance of the date...
EUROPEAN COMMISSION Safety reporting in clinical investigations of medical devices under the Regulation (EU) 2017/745
Safety reporting in clinical investigations of medical devices shall be performed in line with the requirements of the Regulation (EU) 2017/745 – Medical Device Regulation (MDR) Article 80(2): The sponsor shall report, without delay to all Member States in...
EMA preparing big data Q&A guidance
EMA is preparing a question-and-answer guidance document on the impact of EU data protection legislation on the secondary use of health data in support of the development, evaluation and supervision of medicines. The aim is to help medicine developers, data providers...
Medidas excecionais no âmbito da realização de Ensaios Clínicos durante o período de risco para a saúde pública – versão 3
INFARMED 11 mai 2020 Na sequência de emergência de Saúde Pública de âmbito Internacional declarada pela Organização Mundial de Saúde em 30/01/2020 para infeção por SARS-CoV-2 (novo coronavírus 2019), no que se refere à realização de ensaios clínicos em Portugal, o...
Launch of enhanced monitoring system for availability of medicines used for treating COVID-19
EMA, together with the pharmaceutical industry and the EU Member States, has launched its enhanced fast-track monitoring system to help prevent and mitigate supply issues with crucial medicines used for treating patients with COVID-19. Under this system, each...
Trade in Counterfeit Pharmaceutical Products
This report, one in a series of studies by the OECD and the European Union Intellectual Property Office (EUIPO), is designed to enhance understanding of the issues and challenges facing governments, businesses and society posed by the trade in fake pharmaceutical...
Cambridge University :The Future of Medicine
Nanobots that patrol our bodies, killer immune cells hunting and destroying cancer cells, biological scissors that cut out defective genes: these are just some of the technologies that Cambridge University researchers are developing and which are set to revolutionise...
Regulatory flexibility to ensure availability of veterinary medicines during COVID-19 pandemic
The European Commission, EMA and the Coordination Group for Mutual Recognition and Decentralised Procedure – Veterinary (CMDv) have issued guidance on adaptations to the regulatory framework to companies that develop, manufacture and distribute veterinary medicines in...
Union procedure on the follow-up of pharmacovigilance inspections
Date for coming into effect 01 May 2020 This procedure defines the steps in the follow-up of pharmacovigilance inspections and the responsibilities of the parties involved. This includes the process for requesting a CAPA plan in writing from the MAH, CAPA plan review...
New Guidances from European Commission’s Medical Device Coordination Group
The European Commission’s Medical Device Coordination Group (MDCG) on Friday posted five new guidances on demonstrating equivalence to existing devices; clinical evidence for legacy devices; templates for postmarket clinical follow-up plans and evaluation reports; and...