NOTICIAS
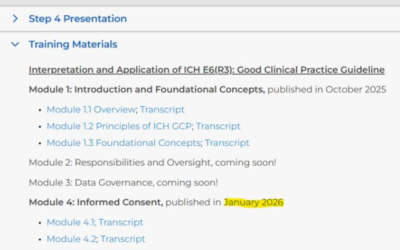
ICH E6(R3) update – New training materials available (Jan 2026)
The ICH Expert Working Group (EWG) has released additional training materials to support the implementation of ICH E6(R3), with a specific focus on Module 4: Informed Consent. Two transcripts are now available: 🔹 Module 4.1 (5 pages) Addresses key questions...
2025 EMA’s key recommendations in human medicines.
EMA have just published the overview of the 2025 EMA’s key recommendations in human medicines. It includes figures on the authorisation of medicines and a selection of new treatments that represent significant progress in their therapeutic areas. ✔️ 104...
Guiding principles of good AI practice in medicine development
EMA and the FDA have jointly identified 10 principles for good artificial intelligence practice in the medicines lifecycle. The use of AI technologies across the medicines lifecycle has increased significantly in recent years and holds great promise as a tool to...
European Pharmacopoeia publishes new data quality framework
The European Pharmacopoeia (Ph. Eur.) has published a new general chapter on Quality of data (5.38) in Issue 12.3, following its adoption by the European Pharmacopoeia Commission at its 182nd session in June 2025. New general chapter on quality of data 5.38 supports...
FDA GUIDANCE DOCUMENT: Clinical Decision Support Software! Guidance for Industry and Food and Drug Administration Staff
This guidance further clarifies that FDA’s existing digital health policies continue to apply to software functions that meet the definition of a device, including those that are intended for use by patients or caregivers. This guidance provides many examples of how...
Use of Bayesian Methodology in Clinical Trials of Drug and Biological Products Guidance for Industry
This document provides guidance to sponsors and applicants submitting investigational new drug applications (INDs), new drug applications (NDAs), biologics licensing applications (BLAs), or supplemental applications on the appropriate use of Bayesian methods in...
The Use of Generative Artificial Intelligence in Research
In this policy brief, team members from Science-Metrix, NIFU and UNU-MERIT (Maastricht University) first conducted a literature review of the current debate on the use of GenAI in research, specifically on the use of chatbots (Section 2). The review is organised...
FDA GUIDANCE ON General Wellness: Policy for Low Risk Devices
The Food and Drug Administration (FDA) is issuing this guidance document to provide clarity to industry and FDA staff on the Center for Devices and Radiological Health’s (CDRH’s) compliance policy for low risk products that promote a healthy lifestyle (general...
IMDRF Strategic Plan 2026-2030
IMDRF is committed to sustaining its strong global leadership while continuing to meet the needs of and engage with its growing number of members and stakeholders. In recent years, IMDRF has led globally in expanding its offerings, now conducting training and...
EMA Medicines – Revised Classification of Changes Guidance
EMA has published its updated “Classification of changes” guidance (Nov 2025) the procedural framework for classifying and submitting variations to marketing authorisations for medicinal products in the European Union. This guidance governs how companies should manage...
EU MDCG Guidance : BtX – Breakthrough Devices under MDR & IVDR
The Medical Device Coordination Group has published MDCG 2025-9 – Guidance on Breakthrough Devices (BtX) under Regulations (EU) 2017/745 (MDR) and 2017/746 (IVDR). This guidance introduces a structured EU framework to support the development, conformity assessment,...
EU MDCG News!! (Post-Market Surveillance Guidance Published)
The Medical Device Coordination Group (MDCG) has released MDCG 2025-10 – Guidance on Post-Market Surveillance (PMS) of Medical Devices and In Vitro Diagnostic Medical Devices (Dec 2025). This document supports manufacturers in understanding and implementing...
Proposal for a regulation to simplify rules on medical and in vitro diagnostic devices
Proposal for a Regulation of the European Parliament and of the Council amending Regulations (EU) 2017/745 and (EU) 2017/746 as regards simplifying and reducing the burden of the rules on medical devices and in vitro diagnostic medical devices, and amending Regulation...
Questions and answers on simpler and more effective rules for medical devices
Why is the Commission proposing a targeted simplification of the Medical Devices Regulations? Medical Devices and in vitro diagnostic medical devices range from sticking plasters, influenza and pregnancy tests to advanced imaging equipment, implants such as hip...
Major proposed changes to MDR & IVDR
The European Commission has published a proposal to amend MDR (EU 2017/745) and IVDR (EU 2017/746), introducing substantial regulatory changes aimed at simplifying compliance, improving predictability, and safeguarding device availability in the EU. The proposal aims...
Artificial Intelligence in Pharmacovigilance
Just published - the most influential guideline for the modern pharmacovigilance today. This report on Artificial intelligence in pharmacovigilance addresses a rapidly emerging cross-disciplinary field that is at the intersection of pharmacovigilance, computer...
European Pharmacopoeia publishes first individual monoclonal antibody medicinal product monograph
The European Pharmacopoeia (Ph. Eur.) has continued to build upon its work in the field of therapeutic monoclonal antibodies with the publication of its first monoclonal antibody (mAb) medicinal product monograph, for the IgG1-antibody based TNF-alpha...
Novas orientações para alterações aos termos da AIM – entrada em vigor a 15-01-2026
A partir de 15 de janeiro de 2026, aplicar-se-á a nova versão das Orientações sobre os pormenores das diversas categorias de alteração relativas a alterações dos termos das autorizações de introdução no mercado
EUDAMED user guide UDI Devices Release 3.022
EUDAMED user guide UDI Devices Release 3.022 Dec 12 2025
FDA Issues Technical Amendments to Align Device Regulations With the New QMSR, Effective February 2, 2026
Regulatory housekeeping with strategic implications: the FDA just updated 179 sections across 21 CFR to reflect the QMSR shift. The FDA has published a new final rule updating references across 21 CFR Parts 801, 803, 812, 860, 862, 864, 866, 868, 872, 874, 876, 878,...
KEY ELEMENTS OF THE EU PHARMACEUTICAL REFORM . December 2025
We welcome the political agreement reached on the pharma reform last night, which is a crucial step in boosting innovation and investment in the EU’s pharma sector. The main aim of the new rules is to make sure that medicines in the EU are safe, effective and...
AI Across the Medicines Lifecycle. Insights from Preliminary Case Studies and Considerations for Policy
Artificial Intelligence is rapidly reshaping the pharmaceutical landscape, from discovery and development to manufacturing and post-approval safety monitoring. As AI becomes more deeply embedded in regulatory decision-making and in processes that directly impact...
HERBAL FOOD SUPPLEMENTS Guide for healthcare professionals
the Committee of Experts on Quality and Safety Standards in Pharmaceutical Practices and Pharmaceutical Care (CD-P-PH/PC), from European Directorate for the Quality of Medicines & HealthCare free practical guide for healthcare professionals ✅ Structured...
FDA eCopy Program for Medical Device Submissions
Document issued December 3, 2025. To comply with the GGP regulations ensuring that regulated entities and the public understand guidance documents as nonbinding, FDA guidance documents ordinarily contain standard language explaining the content to be viewed...
Dezember 20025: key takeaways on the critical medicines act, the pharma package and more
Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL laying a framework for strengthening the availability and security of supply of critical medicinal products as well as the availability of, and accessibility of, medicinal products of...
BOAS PRÁTICAS DE DISTRIBUIÇÃO DE MEDICAMENTOS : “Informativo e esclarecedor “
Decorreu na passada semana uma nova edição da formação BOAS PRÁTICAS DE DISTRIBUIÇÃO DE MEDICAMENTOS , conduzida por Sónia Rei, bajo o lema : "Uma formação essencial para todos os profissionais com atividades GDP que combina a legislação com casos práticos, permitindo...
Study supporting the monitoring of the availability of medical devices on the EU market
The second survey of Economic Operators was just published! Study supporting the monitoring of the availability of medical devices on the EU market Study overview and survey results of the 2nd EO survey with data status 31 October 2024
EAHP 2025 Shortages Survey Report
EAHP released the 2025 Shortages Survey Report, highlighting the ongoing impact of medicines and medical device shortages across European hospitals 📄 Read the full report and key recommendations: https://lnkd.in/ehx4z74s
EUDAMED MILESTONE : Implementation Timelines Confirmed
The European Commission has officially confirmed the functionality of four EUDAMED modules - Actor, UDI/Device, Notified Bodies & Certificates, and Market Surveillance. This marks a significant step forward in the ongoing rollout of the MDR/IVDR regulatory...
Amending Regulations (EU) 2024/1689 and (EU) 2018/1139 as regards the simplification of the implementation of harmonised rules on artificial intelligence (Digital Omnibus on AI)
The Commission is proposing targeted simplification measures to ensure timely, smooth, and proportionate implementation of certain of the AI Act’s provisions. These include: • linking the implementation timeline of high-risk rules to the availability of standards or...
NOTIFICAÇÃO E ALEGAÇÕES de SUPLEMENTOS ALIMENTARES: “Formação muito útil , sobretudo do ponto de vista prático”
Decorreu no dia 18 de novembro a formação NOTIFICAÇÃO E ALEGAÇÕES de SUPLEMENTOS ALIMENTARES conduzida por Rita Dias, Regulatory Affairs Specialist: food and beverage, da PHARMILAB Usando uma combinação de teoria e exercícios práticos, esta formação permitiu...
Artificial intelligence is reshaping health systems: state of readiness across the WHO European Region
Based on an in-depth Member State survey, the report examines six key thematic pillars for AI governance and uptake: national strategies, legal frameworks, data governance, adoption barriers, alignment with health system needs trust building. Together, these...
Formação Qualificação de FORNECEDORES : “Fácil compreensão , muito esclarecedora e muito completa. “
Mais uma edição da formação exclusiva da Formiventos Qualificação de FORNECEDORES ,Clientes e Entidades Subcontratadas, que decorreu na passada quinta feira, conduzida por Teresa Cruz, da MTA Pharma. A formação ofereceu aos participantes uma análise...
FDA GUIDANCE : Patient-Focused Drug Development: Selecting, Developing, or Modifying Fit-for-Purpose Clinical Outcome Assessments
This document provides guidance that is generally applicable to COAs, including patientreported outcome (PRO), observer-reported outcome (ObsRO), clinician-reported outcome (ClinRO), and performance-based outcome (PerfO) measures. Appendices A, B, C, and D include...
ICH E2D(R1) Guideline on post-approval safety data: definitions and standards for management and reporting of individual case safety reports
It is important to establish an internationally standardised procedure to ensure the quality of postapproval safety information and to harmonise, where feasible, the way of gathering and reporting information. The ICH E2D guideline provides guidance on definitions...
PHARMACOVIGILANCE NEWS : Incident management plan
Revision of the Incident Management Plan for Medicines for Human Use (IMP‑H) The EMA has updated its Incident Management Plan for Medicines for Human Use , strengthening how the European medicines regulatory network identifies, assesses, communicates, and escalates...
GDP Nível AVANÇADO : “Formação extremamente enriquecedora”
Dada a crescente procura por competências avançadas em BOAS PRÁTICAS DE DISTRIBUIÇÃO de Medicamentos e substâncias ativas , teve lugar na semana passada mais uma edição da formação exclusiva da Formiventos GDP Nível 2 : BOAS PRÁTICAS DE DISTRIBUIÇÃO Nível Avançado...
Recertification under the MDR and IVDR – Frequently Asked Questions
A new document, explores the challenges associated with the five-year re-certification requirement under the EU Medical Devices Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR). The paper highlights that continuous monitoring, audits, and post-market...
Data Integrity — Frequently Asked Questions (FAQ). Version 3
This document contains a collection of frequently asked questions that have been submitted by the industry to the DI taskforce. The intention of this documents is that this is a living document that will be updated as new questions are opposed to the group.
ISO 13485:2016 Confirmed. – No Revision for Now!
ISO 13485:2016 Confirmed - No Revision for Now! ISO 13485:2016 has officially been confirmed for another five years, meaning it will remain unchanged until the next systematic review. The current focus now shifts to the revision of the ISO 13485 Handbook, which will...
Risk based approach to Post Market Clinical Follow up (PMCF)
MDR & Medical Device Coordination Group (MDCG) guidance: PMCF and Risk The MDR does foresee a risk-based approach to PMCF. Per Preamble (33), the clinical evaluation process is closely interlinked with risk management, which aims to mitigate any residual clinical...
Questions & Answers on the impact of Mutual Recognition Agreement (MRA) between the European Union and the United States
01 October 2025 EMA/INS/GMP/369445/2024 EMA Inspections Office From now on, EU and FDA regulators can rely on each other’s GMP inspections not only within their territories but also outside the EU and US; a huge leap towards global inspection efficiency and reduced...
Digital label for authorised representative and importer
Mandatory requirements for additional product information for medical devices (MDs) and in vitro diagnostic medical devices (IVDs) have exponentially increased recently. Under the current legislative system in the EU, both essential information (information needed...
MANUAL PARA REGULARIZAÇÃO DE EQUIPAMENTO MÉDICO E SOFTWARE COMO DISPOSITIVO MÉDICO NA ANVISA
The regularization of medical equipment and software as a medical device at ANVISA is a crucial process for ensuring safety and efficacy in healthcare technology. The Management of Medical Equipment Technology, known as GQUIP, plays a vital role in this regulation....
How to Prepare a PreRequest for Designation (Pre-RFD) . FDA Guidance for Industry
The FDA just issued final revised guidance explaining how sponsors can get preliminary, non-binding FDA feedback on a product’s regulatory identity (drug, device, biologic, or combination) and Center assignment (CDER, CDRH, or CBER). It replaces the Feb 2018 version...
Formação exclusiva Formiventos CTD MODULO 3: “Evento de alta qualidade que permitiu grande interação entre todos os participantes. “
Nesta semana decorreu mais uma edição da formação exclusiva da Formiventos CTD MODULO 3, bajo o lema : Uma revisão exaustiva de todos os pontos críticos a considerar para garantir o cumprimento dos requisitos regulamentares e atingir os melhores prazos de resposta...
PREÇOS 2025: “Muito interessante e enriquecedora”
No passado dia 31 de outubro decorreu em Lisboa a 6 edição da formação exclusiva da Formiventos : Sistema de Preços de Medicamentos de Uso Humano no SiNATS: Formação, Alteração e Revisão de Preços,conduzida por Jalmira Mulchande, Founding Partner and General...
GUIDANCE DOCUMENT Alternative Tools: Assessing Drug Manufacturing Facilities Identified in Pending Applications
FDA publishes final guidance on Alternative Assessment Tools for Pharmaceutical Manufacturers 📅 09/2025 The Food and Drug Administration (FDA or Agency) is announcing the availability of a final guidance for industry entitled “Alternative Tools:...
FDA Releases Draft Guidance on Quality Management System (QMS) Requirements for Premarket Submissions (October 2025
The Food and Drug Administration (FDA or agency) is issuing this draft guidance to provide guidance to industry and FDA staff about the expectations for quality management system regulation (QMSR) requirements for premarket submissions once the final rule amending 21...
FARMACOVIGILÂNCIA avançada: ““Excelente! Organizada e abrangente !”
Decorreu no dia 28 de outubro a formação exclusiva da Formiventos FARMACOVIGILÂNCIA avançada , desenhada para consolidar e atualizar conhecimentos e permitir o reforço dos sistemas implementados nas organizações “Excelente! Organizada e abrangente ! Foi bom parar...
Study on the deployment of AI in healthcare Final report
Present day healthcare systems face several complex challenges, including rising demand due to an aging population, increasing prevalence of chronic and complex conditions, rising costs, and shortages in the healthcare workforce. Artificial intelligence (AI) has the...